What is electroplating?
Electroplating is a finishing process used in various industries to enhance the properties of metal objects. It involves the application of a thin layer of metal onto the surface of another metal through an electrochemical procedure. Initially, metals could only be electroplated with other metals, but with recent technological advances, non-metals can also be improved with this process.
At its core, electroplating uses an electric current to reduce dissolved metal cations so that they form a coherent metal coating on an electrode, typically the object that requires plating.
The process begins with the creation of an electrolytic cell, which comprises two essential components: the anode, or the positive electrode, and the cathode, or the negative electrode. These electrodes are submerged in an electrolyte solution that contains ions of the metal to be plated. When electricity is applied, it drives a reaction where metal ions from the solution are deposited onto the cathode, gradually building up a layer of the plating metal.
Electroplating is a valuable surface finishing technique for industries ranging from jewelry and automotive manufacturing to electronics and aerospace engineering.

Image Source: Formlabs
Electroplating Process
Preparation of the Substrate
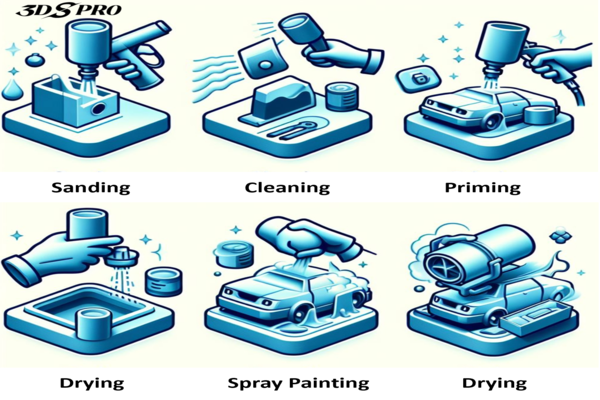
Before electroplating can begin, the substrate – which is the object to be plated – must be thoroughly cleaned, involving removing any dirt, grease, or oxidation to ensure that the metal coating will adhere properly.
Bath Setup
An electrolyte bath is prepared, which is a solution containing the metal ions of the plating metal. The substrate is then immersed in this bath and connected to the negative terminal of a power source, becoming the cathode.
Anode and Cathode Connection
A metal bar of the plating material is connected to the positive terminal of the power source, serving as the anode. This bar will slowly dissolve, providing the metal ions needed for plating.
Application of Electric Current
Once everything is in place, an electric current is applied. The current causes the metal ions in the bath to move toward the negatively charged substrate and adhere to its surface.
Layering
The thickness of the metal layer can be controlled by the duration of the electroplating process and the strength of the electric current. The longer the process runs, the thicker the metal coating.
Post-Plating Treatment
After the desired thickness is achieved, the substrate is removed from the bath. It may undergo additional treatments such as rinsing, drying, and polishing to enhance the quality of the coating.
Quality Inspection
The final step is to inspect the plated object for uniformity, adhesion, and thickness to ensure it meets the required specifications.
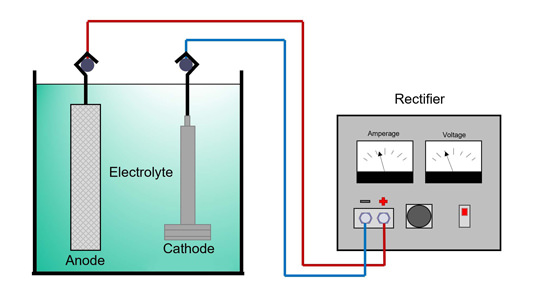
Image Source: US Chrome
Benefits of Electroplating
1. Enhanced Durability: Electroplating adds a protective barrier that increases the lifespan of components by resisting wear and corrosion.
2. Improved Aesthetics: A plated finish can significantly improve the appearance of an object, making it more appealing and marketable.
3. Reduced Friction: Certain electroplating materials, like nickel, can decrease friction, leading to better performance and less wear.
4. Superior Electrical Conductivity: Metals like silver, when used in electroplating, can enhance electrical conductivity, which is crucial for electronic components.
5. Heat Resistance: Some electroplating processes enable components to withstand extremely high temperatures, protecting them from heat-related damage.
6. Magnetic Properties: Electroless nickel plating, for example, is used in magnetic applications, such as in the manufacturing of computer hard drives.
7. Increased Hardness: Electroplating can strengthen materials, making them more robust and less prone to damage upon impact.
8. Light and Energy Absorption: Certain types of electroplating can absorb light and energy, which is vital in industries like aviation and aerospace.

Image Source: Sharretts Plating
Limitations of Electroplating
1. Complexity: The electroplating process can be intricate and challenging to execute consistently, requiring precise control over various parameters.
2. Cost: Electroplating can be expensive, especially for large or complex objects, due to the materials and energy required.
3. Environmental Impact: The process can generate hazardous waste and byproducts that must be managed properly to avoid environmental harm.
4. Thickness Limitations: Electroplating typically involves very thin metal layers, which may not provide a thick shell for certain applications.
5. Material Restrictions: Not all materials can be electroplated, and the choice of metals for plating can be limited by factors such as compatibility and cost.

Image Source: KEYENCE
Types of Electroplating Methods
Mass Plating
Mass plating is also known as barrel plating. This method is suitable for plating a large volume of small components. The parts are placed in a rotating barrel that is immersed in the plating solution, allowing for even coating and efficient processing.
Rack Plating
Rack plating involves affixing parts onto a rack before submerging them into the plating bath. Rack plating is ideal for larger or more delicate parts that require a uniform finish.
Continuous Plating
Continuous plating is used for wire, strip, or tubing, continuous plating involves running the substrate through a continuous sequence of plating baths. It’s an efficient method for high-volume production.
In-line Plating
In-line plating is similar to continuous plating, in-line plating is a streamlined process where parts move through various pre-treatment and plating stages without interruption, often part of an automated production line.
Direct Electroplating
Direct electroplating involves the direct deposition of metal ions onto the object to be plated. It’s the most straightforward approach and is commonly used for a variety of applications.
Indirect Electroplating
In indirect electroplating, metal ions are first deposited onto an electrode, which is then placed in contact with the object. This method can be used for specialized plating requirements.

Image Source: Techmetals
Electroplating Material Options
Gold
Gold is known for its excellent conductivity and resistance to tarnishing. It is often used in high-end electronic and aerospace applications.
Silver
Silver is another highly conductive metal that also provides anti-tarnishing properties. It’s frequently used in electrical components.
Nickel
Nickel plating is popular for its hardness and durability, offering wear and corrosion resistance. It’s widely used in automotive and industrial parts.
Copper
Copper is chosen for its superior electrical conductivity and is often used as an undercoat before other metals are plated.
Zinc
Zinc is favored for its ability to protect against corrosion, making it ideal for coating fasteners, steel parts, and automotive components.
Chromium
Chromium plating is used for its high hardness and shiny finish, often seen in decorative applications as well as in durable coatings.
Titanium
This metal is valued for its strength-to-weight ratio and corrosion resistance, suitable for medical devices and aerospace components.
Brass
Brass electroplating is chosen for its appearance and antimicrobial properties, commonly used in fixtures and musical instruments.
Cadmium
Although less common due to environmental concerns, cadmium offers excellent corrosion resistance and is used in aerospace applications.
Iron
Iron plating is utilized for its magnetic properties and is often found in hard disk drives and other magnetic storage devices.

Image Source: Valence Surface Technologies
Applications of Electroplating
1. Aerospace: In the aerospace industry, electroplating is crucial for improving the strength, durability, and corrosion resistance of aircraft components. It is used on engine parts, landing gear, and even cabin fixtures to ensure they can withstand the extreme conditions of flight and atmospheric exposure.
2. Automotive: The automotive sector relies on electroplating for both functional and decorative purposes. Components like gears, bearings, and fasteners are electroplated to resist wear and corrosion. Decorative trims and emblems are also electroplated to enhance appearance and durability.
3. Electronics: Electroplating is essential in the electronics industry for creating conductive pathways and connections. It is used in the manufacturing of semiconductors, connectors, and other critical electronic components.
4. Medical Devices: In medical technology, electroplating is used to produce biocompatible surfaces on devices that come into contact with the human body, such as surgical instruments and implants.
5. Defense: Military equipment and vehicles benefit from electroplated coatings that provide enhanced protection against environmental and operational wear.
6. Energy: Electroplating is employed in the energy sector to coat components of power generation systems, such as turbines, to improve efficiency and resistance to harsh conditions.
7. Consumer Goods: From household appliances to personal gadgets, electroplating is used to apply a protective and attractive finish to a wide range of consumer products.
8. Space Exploration: Components used in space missions are often electroplated to endure the vacuum of space, extreme temperatures, and radiation exposure.
Image Source: Aerospace Metals
3DSPRO Electroplating 3D Printed Resin and Metal Parts
3DSPRO integrates electroplating surface finishing with 3D printing, offering advanced solutions for both resin and metal printed parts. We provide customized electroplating services tailored to the unique or specific requirements.
Whether it’s for aesthetic purposes or functional use, 3DSPRO ensures a high-quality finish that enhances the appearance and performance of the electroplated 3D prints.
We are committed to sustainable practices, minimizing waste and environmental impact by optimizing the electroplating processes and using eco-friendly materials when possible.
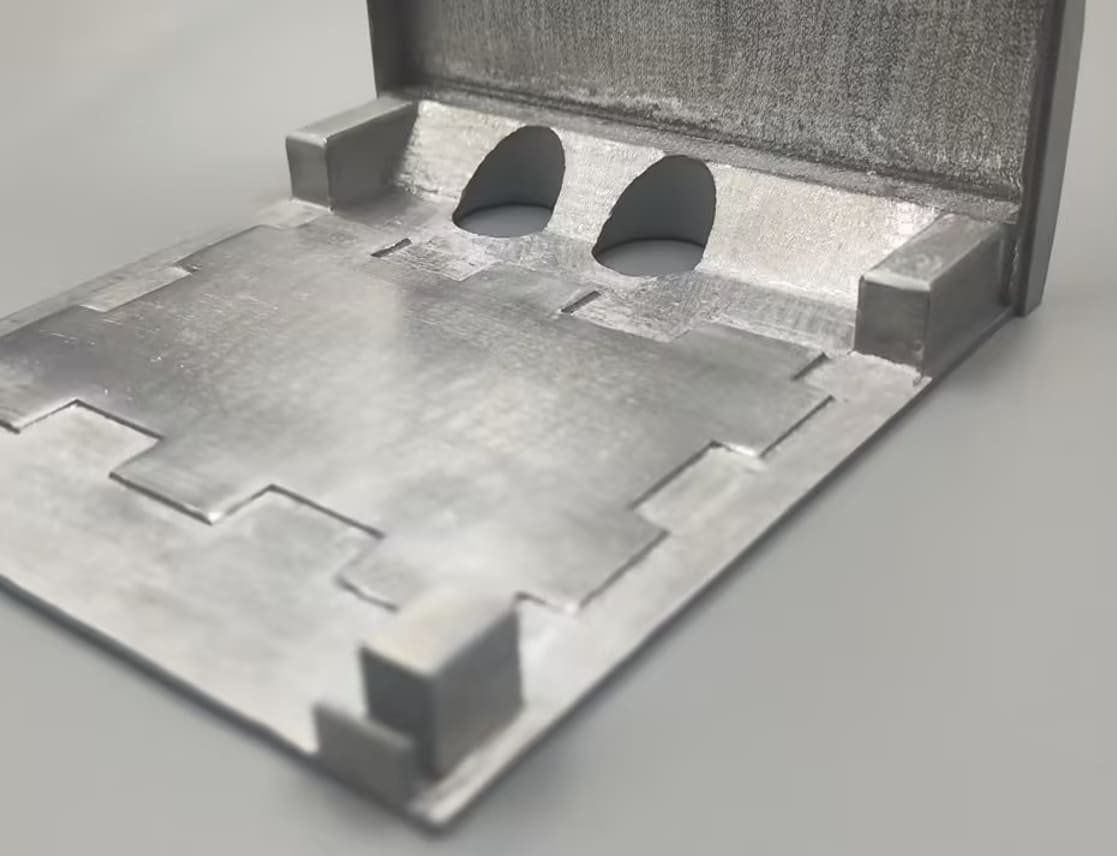
3DSPRO Electroplated 3D Printed Aluminum Sample:

COMMENTS
- Be the first to share your thoughts!